Patent MarketPlace: Pharmaceutical Patents for Sale
 Nutritional Compound for Gut-Brain Weight Loss (Dellinger): U.S. Patent Nos. 11,771,125 and 11,813,363
Nutritional Compound for Gut-Brain Weight Loss (Dellinger): U.S. Patent Nos. 11,771,125 and 11,813,363
With soaring healthcare costs and an emergent chronic disease pandemic associated with obesity, there is a global need for an affordable, safe alternative to high-priced, often unreimbursed pharmaceutical solutions with limited outcomes and daunting side effects. This patent covers a nutraceutical formulation that delivers the amino acids of tryptophan to the distal end of the intestines where they convert into the appetite-appeasing neurotransmitter, serotonin. Leveraging digestive biology and vagus neuron-signaling, this innovation naturally stimulates GLP-1 hunger suppression as a safe, affordable option to prescription drugs. It is a supplement to a single, unrestricted meal each day – free of quantity, caloric, or food group requirements.
This patent family covers a safe and sustainable weight-loss regimen that consists of natural nutrients from milk and eggs – specifically formulated and engineered for precision intestinal assimilation and synaptic communication between the gut and brain. It is designed to be taken along with one meal a day for steady, consistent, no-side-effects, weight loss that is convenient and affordable for the individual. The formulation delivers precise signaling molecules to specialized cells located at the distal end of the small intestines. This signal-cell interaction sends satiety signals to the brain. In addition to weight loss, the formulation improves daily cognitive function, improves satiety hormones, enhances gastric well-being, and could potentially reduce glucose and insulin levels. Early clinical feasibility studies revealed success with results pending peer review in a leading nutrition and obesity scientific journal.
U.S. Patent Nos. 11,771,125 and 11,813,363 for a “Concentrated nutritional or supplemental compound for intestinal, gut-brain axis and neurobiological homeostasis through calibrated absorption including neurotransmitter or any equilibrating compound release to treat or mitigate disease and co-morbidities, particularly obesity and malnourishment” would be a strategic acquisition for any nutraceutical supplements supplier that is ready to introduce a 100% natural weight-loss regimen!
 SIRP-Based Immunotherapy (OmniAb): International Patent Portfolio
SIRP-Based Immunotherapy (OmniAb): International Patent Portfolio
One of the most promising cancer treatments is immunotherapy. It uses substances made by the body or in a laboratory to boost the immune system, helping the body find and destroy cancer cells. Immunotherapy can treat many different types of cancer, and it can be used alone or in combination with chemotherapy and/or other cancer treatments.
Signal regulatory proteins (SIRPs) are a family of immune receptors encompassing inhibitory, activating, nonsignaling and soluble members. CD47, a broadly expressed transmembrane glycoprotein, functions as a cellular ligand for SIRPα and γ, negatively regulating phagocytosis and antibody-directed cellular cytotoxicity (ADCC). Agents that interfere with the interaction between these SIRPs and CD47 thus activate phagocytosis and ADCC. This portfolio describes SIRPγ and SIRPβ/β2 decoy polypeptides for immunotherapy treatment of cancer, anemia, transplant rejection, asthma, allergies, auto-immune disease, and viral infection. These decoy polypeptides are variants comprising at least one amino acid modification to increase affinity of the decoy polypeptide binding to CD47.
International Patent Portfolio
- U.S. Patent No. 9,845,345: SIRP polypeptide compositions and methods of use
- U.S. Patent No. 10,774,125: SIRP-gamma polypeptide compositions and methods of use
- U.S. Patent No. 11,407,801: SIRP-gamma polypeptide compositions and treatment of cancer
- European Patent 3298043: SIRP polypeptide compositions and methods of use
- China Patent 107849143: SIRP polypeptide compositions and methods of use
- Japan Patent 7064234: SIRP polypeptide composition and method of use
This portfolio will enable any provider of cancer treatments or immunotherapy drugs to introduce a new generation of polypeptide-based signal regulatory proteins.
 Molecular Disruption for Disease Transmission Mitigation (Dellinger): Patent No. 11,638,720
Molecular Disruption for Disease Transmission Mitigation (Dellinger): Patent No. 11,638,720
Our environment is laden with infectious agents, and the recent global health crisis has intensified awareness of our vulnerability to diseases that can be both pervasive and lethal. Society is constantly at risk from various infections and the rise of antimicrobial resistance (AMR). In the arms race against these evolving pathogens, scientific innovation must stay a step ahead to develop safer, more effective solutions.
This patent introduces an advanced, broad-spectrum antimicrobial solution poised to redefine infection control. The composition utilizes atomic-scale nanoparticles modified with caustic halogens that annihilate pathogens of all varieties, including viral, bacterial, and fungal species. Activated by aqueous solutions, the composition ensures deep microbial clearance without harming human tissues, setting a new precedent in infection prevention, and offering unparalleled sterility and safety. Designed for comprehensive disinfection of skin, mucosa, and internal regions, this innovation represents a leap forward in personal protection, hygiene, and risk mitigation against infectious diseases. Additional applications covered by this patent include an aqueous suspension that employs diuresis to flush the urinary tract, potentially preventing both urinary tract and sexually transmitted infection colonization. Potential clinical applications are vast and vital, including pre-surgical sterilizing scrubs, injections, phlebotomy, or IV therapy as preventive measures against nosocomial infections. This novel approach could revolutionize the prevention and treatment of various infections, giving the healthcare sector an entirely new tool in its fight against infectious diseases and AMR.
U.S. Patent No. 11,638,720 for a “Risk mitigation of infectious disease transmission from incidental and intimate contact using atomic scale molecular disruption and biocidal halo-fullerenes delivered via topical, flushing and enteral mechanisms” can be used to create the next generation of biocides to fight viral, bacterial, and fungal infections.
 Longer Viability for Transplant Organs (Brady): U.S. Patent No. 11,452,288
Longer Viability for Transplant Organs (Brady): U.S. Patent No. 11,452,288
Organ transplants save lives and restore sight to tens of thousands of Americans each year. And while there are over 100,000 Americans on waiting lists for donor organs, more and more Americans are signing up to donate their organs. A key challenge facing the organ transplant community is getting a donated organ to the recipient before the organ has deteriorated and is not usable. What an incredible tragedy it is when an organ is donated, but by the time it arrives for transplant it is no longer viable!
This patent covers an innovative approach to preserving donor organs. Hemocyanin, such as that found in the Atlantic horseshoe crab (Limulus polyphemus), will deliver oxygen directly to transplant tissues while free radical scavenging by pristine fullerenes helps maintain cellular integrity and mitigates degradation caused by oxidative stress. Horseshoe crabs have successfully evolved over millions of years by developing unique immune cells called amoebocytes. These cells have a specific and highly sensitive affinity to gram-negative bacteria and fungi – engulfing these microbes in a destructive enzymatic coagulation process. The viral-scale peptides produced by the horseshoe crab’s amoebocytes invade pathogens and disrupt replication without conferring a caustic or toxic effect on the non-pathogenic tissue. This patent creates a hemocyanin-based formulation from horseshoe crabs that is added to tissue storage and preservation media as a potent, broad-spectrum, antimicrobial supplement.
U.S. Patent No. 11,452,288 for an “Innocuous sterilant using hemocyanin and functionalized fullerenes with broad-spectrum intracellular and interstitial microbiocidal and radical scavenging effects for packaged matter, biologics and organics including liquids, gases, tissue, organs, cells, and limbs with copper mediated oxygenation for viability and preservation” will enable any biotechnology or pharmaceutical company to introduce a formulation that will dramatically improve the shelf-life of donated organs, saving or transforming even more lives.
 Mini-Tablet Dispenser (Balda Medical): International Patent Portfolio
Mini-Tablet Dispenser (Balda Medical): International Patent Portfolio
The new mini-tablets in which many drugs are being prescribed – drugs for leukemia, epilepsy, and heart disease are just three – combine the advantages of solid and liquid formulations. They are easy to swallow and provide an accurate dosage, but do not carry the risk of over- or under-dosage that liquids present.
What is needed is a dispenser specifically for this new generation of mini-tablets, and that what this international patent portfolio creates. The dispenser covered by this portfolio stores and dispenses one mini-tablet at a time without breaking or crushing tables in the process. It has an activation lock to prevent misuse. Its rotational design insures controlled dosage of the mini-tablets it holds and it provides visual control of the dosage. The unit is easily locked to secure the tablets, and it is compact enough to be easily stored in a pocketbook.
Patent Portfolio
- U.S. Patent No. 10,099,841: Dispensing device for solid portions and method for dispensing solid portions
- European Patent 2855303: Dispensing device for solid portions and method for dispensing solid portions (Valid in Switzerland, Germany, Spain, France, Great Brittain, and Italy)
- U.S. Patent No. 9815611: Device and method for singularized dispensing of solid portions
- European Patent 3016885: Device and method for dosing pill-shaped elements (Valid in Switzerland, Germany, Spain, France, Great Brittain, and Italy)
- India Patent 444126: Device and method for dosing pill-shaped elements
Any manufacturer of pharmaceutical packaging could use this portfolio to introduce the first packaging product specifically designed to meet the needs of those with a prescription drug in mini-tablet form.
 Opioid Medications to Prevent Overdose or Death During Treatment (Presti): International Patent Portfolio
Opioid Medications to Prevent Overdose or Death During Treatment (Presti): International Patent Portfolio
The U.S. Department of Health and Human Services reports that deaths from drug overdose have risen steadily over the past two decades to the point that they have become the leading cause of deaths from injury in the United States. Deaths from opioid overdoses have surpassed automobile accident fatalities, the leading cause of injury deaths in U.S. for the last century. Worse yet, many opioid addicts enter treatment, but then overdose during treatment when they self-medicate with alcohol. According to the Centers for Disease Control (CDC), nearly one quarter of fatal prescription opioid overdoses in the U.S. are associated with patients making the all-too-common mistake of drinking alcohol while medicated. When combined, alcohol and opioids interact in the brain increasing the risk of respiratory depression and death.
This portfolio aims to prevent opioid deaths head-on by solving this critical, but previously unaddressed, dimension of the opioid crisis. It includes several combination drug therapies that reduce the likelihood of an alcohol-mediated opioid overdose or death during maintenance therapy for management of pain or opioid use disorder. It covers a single composition comprising an effective amount of one or more opioid medications and an effective amount of one of 20 aldehyde dehydrogenase inhibitors or one of 65 opioid medications. It also includes a combination medication comprised of an effective amount of one or more opioid medications, an effective amount of one or more aldehyde dehydrogenase inhibitors, and a pharmaceutical carrier.
This extensive portfolio additionally includes a combination medication that is comprised of one or more opioid medications from 1 to 200 mg, a disulfiram or pharmaceutically acceptable salt from 50 to 250 mg, and a carrier. Specifically directed at reducing an alcohol-mediated opioid overdose or death during maintenance therapy for pain management, the portfolio covers a single composition comprised of one or more opioid pain medications from 1 to 200 mg and a disulfiram or pharmaceutically acceptable salt from 50 to 250 mg that prevents alcohol consumption in the patient. There are additional formulations in three pending patent applications.
Patent Portfolio
- U.S. Patent No. 10,478,408: Combination treatments for opioid crisis
- U.S. Patent No. 10,881,625: Combination treatments for opioid crisis
- U.S. Patent No. 11,786,490: Combination treatments for opioid crisis
- U.S. Patent Application 20220000809: Combination treatments for opioid crisis
- U.S. Patent Application 20220117916: Combination products to mitigate the risk of non-benzodiazepine benzodiazepine against adverse reaction and overdose
- European Patent Application 3743073: Combination treatments for opioid crisis
- Canadian Patent Application 3089071: Combination treatments for opioid crisis
- Indian Patent Application 202047035537: Combination treatments for opioid crisis
- Mexican Patent Application 2020007860: Combination treatments for opioid crisis
- PCT Patent Application 2023015455: Combination products to mitigate misuse of central nervous system stimulants
This portfolio offers a pharmaceutical company the opportunity to introduce the next generation of opioid treatments – a regimen that specifically addresses opioid patients that face severe pains issues during treatment at high risk of drinking alcohol during their treatment for pain or addiction.
 Nutraceutical Treatment for Gout (Truscott): U.S. and European Patents
Nutraceutical Treatment for Gout (Truscott): U.S. and European Patents
Gout is a form of inflammatory arthritis that is caused by excess uric acid in the blood that crystallizes in joints and other tissues. Among those with gout, 90% have kidneys that don’t adequately remove uric acid from their urine, while 10% have high uric acid levels because their bodies produce too much uric acid. Between eight and nine million Americans suffer from gout – and millions more in Europe. Gout can be very painful – even disabling. Most treatments for gout address the pain and inflammation caused by the condition, not the root cause of the disease!
This portfolio covers a safe and effective treatment for gout that is composed of natural ingredients – celery seed extract, cherry extract, and elemental lithium in a lithium salt. The components work synergistically to inhibit painful uric acid crystals by three different modes of action: Inhibition of uric acid formation (celery seed extract), increase in uric acid elimination from the body (cherry extract), and interference with crystallization itself (lithium). Since the ingredients are all-natural, a gout treatment based on these patents can be sold over-the-counter without a prescription.
U.S. Patent No. 9,149,500 and European Patent 2303302 for a “Method and process for relieving or preventing symptoms” would enable any pharmaceutical supplier or natural remedy manufacturer to bring a treatment for gout to the market very quickly!
 Urinary Tract Infection Prevention and Treatment (Biomedicals Science Sweden): International Patent Portfolio
Urinary Tract Infection Prevention and Treatment (Biomedicals Science Sweden): International Patent Portfolio
In the science fiction thriller The Andromeda Strain, scientists attempting to stop an infection from outer space discover that the disease can only survive in a narrow pH range. The same is true of many infections here on earth including bacteria that can make its way up the urinary tract to infect the bladder (causing cystitis) or kidneys (causing pyelonephritis). Controlling the pH level in the patient’s urinary tract can prevent bacteria from becoming an infection.
This portfolio covers alternating administrations of a pH-increasing agent with the administration of a pH-decreasing agent to the patient via a mannose solution. The pH-increasing agent is administered at a dosage of about 0.1 to 20 grams per day, while the pH-decreasing agent is administered to at a dosage of about 0.1 to 20 grams per day, and the mannose or other solution is administered to at a dosage of about 0.1 to 50 grams per day.
Urinary Tract Infection Portfolio
- U.S. Patent No. 10,525,069: Treatment of urinary tract infection
- European Patent 3164138: Treatment of urinary tract infection
- China Patent 107073018: Treatment of urinary tract infections
- Sweden Patent 538140: Treatment of urinary tract infection
- Spain Patent 2880794: Urinary tract infection treatment
- Denmark Patent 64138: Treatment of urinary route infection
This portfolio can be developed into a product that is sold over-the-counter in pharmacies or is used in physician’s offices as a pre-treatment prior to prescribing antibiotics. It can also be used in hospitals after surgery to lower the risk of infection and as a product for catheter patients, significantly lowering the use of antibiotics among this high-risk infection group. This portfolio will enable any drug manufacturer to introduce a product that totally prevents, or very quickly and effectively treats, a urinary tract infection without the use of antibiotics and their resulting side effects.
 Treatment for Afflictions of the Central Nervous System (Numazawa): U.S. and Japanese Patents
Treatment for Afflictions of the Central Nervous System (Numazawa): U.S. and Japanese Patents
It is not uncommon for a drug that was originally developed to treat one disease or condition to be found to also treat another disease or condition. For example, minoxidil, the active ingredient in Rogaine® (the popular treatment for male pattern baldness) was originally developed to treat high blood pressure! This is known as “drug repositioning.”
This patent portfolio addresses new uses for two existing drugs – Methocarbamol and Chlorphenesin Carbamate – for the treatment of central nervous system afflictions such as mood disorders, mental disorders, chronic fatigue syndrome, depression, and Alzheimer’s. The inventor has three published medical research papers documenting the effectiveness of this patented drug combination.
This portfolio includes U.S. Patent No. 11,364,218 for a “Method of treating or preventing mood disorders, mental disorders, and/or chronic fatigue syndrome” and Japanese Patent 6216913 for a “Pharmaceutical composition.” Since the two drugs on which the patents are based are already FDA-approved, the acquirer of the patents should be able to go to market with a drug for the treatment of depression and Alzheimer’s fairly quickly.
Rogaine is a registered trademark of Johnson & Johnson Services, Inc.
 Production of Very Pure HMR and NTG Lignans (Montisera): International Patent Portfolio
Production of Very Pure HMR and NTG Lignans (Montisera): International Patent Portfolio
Lignans are a little-known group of low molecular weight polyphenols that are derived from trees and other ligneous plants. HMR (hydroxymatairesinol) lignans are a powerful antioxidant and immune system booster that is used in by humans as well as dogs and cats. It is also an enterolactone precursor with anticancer activities. Testing has shown that HMR lignans decrease the volume of induced tumors and stabilize established tumors – and prevent the development of new tumors – in rats. HMR lignans and NTG (nortrachelogenin) lignans are also key ingredients in natural cosmetics that offer anti-aging properties. Research has shown promise for NTG lignans in treating prostate cancer.
The challenge is refining lignans through the fractionation of knotwood extract. This portfolio covers a process that in which hydrophilic extract is extracted with a lipophilic solvent that removes lipophilic impurities. The portfolio also covers the use of liquid-to-liquid extraction for the purification of hydrophilic knotwood extract. The patented process provides a purified extract that contains more than 90 % lignans, flavonoids, and stilbenes, and less than 10 % in impurities.
Patent Portfolio
- European Patent 2310379: Method for the fractionation of knotwood extract and use of a liquid-liquid extraction for purification of knotwood extract (valid in Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, and UK)
- Finnish Patent 123498: Method for fractionation of tree tree extract and use of liquid-liquid extraction to purify tree extract
- Canadian Patent 2728934: Method for the fractionation of knotwood extract and use of a liquid-liquid extraction for purification of knotwood extract
- U.S. Patent No. 9,919,306: Dosing pipette
- U.S. Patent RE46721: Method and device for the metered dispensing of a medium
- European Patent 2934753: Dosing pipette (France, Germany, and Italy)
- European Patent 2120465: Method and device for the metered dispensing of a medium (France, Germany, Great Britain, Italy, Spain, Switzerland)
- Brazil Patent 112015014341: Dosing pipette
- Canada Patent 289528: Dosing pipette
- China Patent 104994958: Dosing pipette
- German Patent Application 102013114336: Dosing pipette
- U.S. Patent No. 10,751,358: Multitargeted nucleoside derivatives
- European Patent Application 3618837: Multitargeted nucleoside derivatives
- Chinese Patent Application 110831605: Multi-targeted nucleoside derivatives
- Japanese Patent Application 2020518657: Multi-targeted nucleoside derivatives
- Canadian Patent Application 3061621: Multitargeted nucleoside derivatives
- Brazilian Patent Application 112019022470: Compound, composition, and, method for treating an individual with cancer
- U.S. Patent No. 9,283,150: Pill dispensing system
- U.S. Patent No. 10,653,584: Tablet and capsule dispensing assembly
- U.S. Patent No. 10,772,805: Tablet and capsule dispensing assembly
- U.S. Patent No. 11,053,065: Tablet and capsule dispensing assembly
- U.S. Patent No. 11,116,698: Method of installing and removing a rotation mechanism within pill dispensing assemblies
- U.S. Patent No. 8,815,898: 6,7-disubstituted-sioquinoline derivatives and their use
- European Patent 2603217: Novel 6,7-disubstituted-isoquinoline derivatives and their use (28 national patents)
- Canada Patent 2808016: 6,7-disubstituted-isoquinoline derivatives and their use
- Russia Patent 2636938: Novel 6,7-disubstituted-isoquinoline derivatives and their use
- This new platform of a combination of drugs rebalances the microbial gut and mitigates pathogenic inflammatory triggers.
- In contrast to most antibiotics that dissolve in the stomach with side effects, the capsule covered by this portfolio targets optimal delivery to the areas of highest need.
- The treatment covered by this portfolio mitigates the effects of chronic inflammation often implicated as a causal pathway in the development of colorectal cancer.
- The drugs covered by this portfolio address the underlying causal factors of the disease as opposed to most treatment options that merely manage the symptoms.
- This is a single-dose drug product that is superior to multiple individual pills that result in increased patient inconvenience and non-compliance.
- This patented solution offers clinicians a less toxic, safer, and more effective treatment option than steroids, immunosuppressants, and biologics.
- U.S. Patent No. 9,138,441: Compositions and method for treatment and prophylaxis of inflammatory bowel disease
- U.S. Patent No. 9,649,348: Compositions and method for treatment and prophylaxis of inflammatory bowel disease
- Australian Patent 2010339573: Compositions and method for treatment and prophylaxis of inflammatory bowel disease
- Canadian Patent 2,785,658: Compositions and method for treatment and prophylaxis of inflammatory bowel disease
- Mexican Patent Application 348611: Compositions and method for treatment and prophylaxis of inflammatory bowel disease
- Israeli Patent Application 220613: Use of an antibiotic and probiotic formulation in the preparation of a medicament for treatment of inflammatory bowel disease
- U.S. Patent No. 10,172,772: Methods of skin whitening by use of canola extracts
- U.S. Patent Application 20190374453: Methods of skin whitening by use of canola extracts
- U.S. Patent Application 16/842,686: Methods of skin whitening by use of canola extracts
- Australian Patent 2014210919: Methods of skin whitening by use of canola extracts
- Australian Patent 2019200853: Methods of skin whitening by use of canola extracts
- European Patent Application 2950779: Methods of skin whitening by use of canola extracts
- Canadian Patent Application 2899814: Methods of skin whitening by use of canola extracts
- Korean Patent Application 10-2015-7023569: Methods of skin whitening by use of canola extracts
- Japanese Patent Application 2020-059727: Methods of skin whitening by use of canola extracts
- PCT Patent Application 2014118610: Methods of skin whitening by use of canola extracts
- U.S. Patent No. 9,132,117: Compositions and methods for glycemic control of subjects with impaired fasting glucose
- U.S. Patent No. 9,610,276: Compositions and methods for glycemic control of subjects with impaired fasting glucose
- Australian Patent 2014282942: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- Indian Patent 299738: Use of polymethoxylated flavones for treating insulin resistance
- European Patent Application 3010499: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- PCT Patent Application 2002087567: Use of polymethoxylated flavones for treating insulin resistance
- PCT Patent Application 2014203059: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- Canadian Patent Application 2915751: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- Australian Patent Application 2019200898: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- New Zealand Patent Application 715160: Compositions comprising at least one polymethoxyflavone, flavonoid, liminoid, and/or tocotrienol useful in combination therapies for treating diabetes
- U.S. Patent No 10,507,202: Naproxen-based non-steroidal anti-inflammatory drug with low gastric toxicity
- U.S. Patent No 10,688,084: Novel use of naproxen derivatives
- Chinese Patent Application 109923103: low stomach toxicity non-steroidal anti-inflammatory drugs based on naproxen
- European Patent Application 3517525: Naproxen-based non-steroidal anti-inflammatory drug with low gastric toxicity
- Japanese Patent Application 2019537627: Naproxen nonsteroidal anti-inflammatory drugs with low gastric toxicity
- Indian Patent Application 201927013675: Naproxen-based non-steroidal anti-inflammatory drug with low gastric toxicity
- U.S. Patent No. 9,579,312: Method of treating/preventing disease using cognitive ability of cerebrum and pharmaceutical
- European Patent 2005956: Method of treating/preventing disease using cognitive ability of cerebrum and pharmaceutical
- Japan Patent 4615470
- Hong Kong Patent HK1126659
- PCT Patent Application 200711097
- Executive Summary
- Patent Overview and History
- Technology and Investment Summary
- Market Research
- Company Analysis
- Illustrative Evidence of Use (if applicable)
- Agricultural
- Artificial Intelligence
- Automotive/Vehicular
- Aviation
- Banking/Financial Services
- Beverages/Foods/Nutritional Products
- Boat and Marine
- Cannabis and Medical Marijuana
- Construction/Building Trades
- Consumer Electronics
- Consumer Products
- Digital Currency/Cryptocurrency
- Drones/UAVs
- E-Cigarette & Vaping Technology
- E-Commerce
- Education & Training
- Energy/Power Generation
- Health and Beauty Products (HBP)
- Human Resources
- IoT Patents/Internet of Things
- Manufacturing
- Medical Electronics and Devices
- Mining/Drilling
- Mobile/Wireless
- Network/Location-Based Services
- Optics/Displays/Video/LED
- OTT Patents/Over-the-Top
- Packaging
- PCs and Notebooks
- Pharmaceuticals
- Robotics/Automation
- Semiconductor
- Shoe & Apparel
- Smart Home/Smart Office
- Social Media
- Software, Apps, and Architecture
- Sports/Sporting Goods
- Telecommunications/IP Telephony
- Warehousing/Material Handling
- Other
- Go to Patent Index
- Return to Patent MarketPlace
This portfolio covers a manufacturing process model that will enable any business that uses lignans in its products to more efficiently refine high quality lignans from knotwood extract, decreasing production costs and increasing product quality.
 Exact Dosage Liquid Medicine Dispenser (Balda): International Patent Portfolio
Exact Dosage Liquid Medicine Dispenser (Balda): International Patent Portfolio
Administering exactly the correct dose of a liquid medicine – prescription or over-the counter – can be tricky. Especially in children, the correct dosage of cough medicine, pain killer, or other liquid drug is very important to prevent over-dosing. Pouring the liquid into a measuring cup is messy, inaccurate, and wasteful. There has to be a better, more accurate way to orally administer a liquid drug.
This portfolio addresses that challenge be creating a pipette that has rachets on the inside wall of the barrel at set intervals (every 0.5 ml, every 1.0 ml, for example) that are marked on the housing rim so the user can determine which rachet is the desired dosage. As the user draws back on the plunger, he or she can feel it pass each rachet in the barrel of the pipette. This is known as “haptic” or “tactile” feedback. The user receives feedback from the plunger as it passes each rachet on the inside of the barrel. Once the user has drawn exactly the correct dosage of liquid into the pipette, he or she depresses the plunger to its original position to deliver the liquid into the patient’s mouth. Since the tip of the piston is located at the tip of the barrel, there is almost no unadministered medicine left in the pipette. Additionally, the pipette does not come in direct contact with the recipient of the liquid drug, so it cannot become contaminated.
Patent Portfolio
The pipette covered by this portfolio consists of just two components, the barrel and the plunger, so it is very economical to manufacture, and the design is totally scalable based on the range of doses that are given for a particular liquid drug. This portfolio will enable any drug manufacturer or drug packaging manufacturer to bring to market the first major innovation in liquid medications in a century!
 DNA-Altering Cancer Treatment (Kalman): International Patent Portfolio
DNA-Altering Cancer Treatment (Kalman): International Patent Portfolio
Among the over 1,000 cancer-fighting drugs on the market today, two of them are Capecitabine and Gemcitabine. Capecitabine is sold under the Xeloda® brand is orally active. It is used primarily to treat breast cancer, and it works by blocking DNA synthesis and causing the loss of integrity of DNA. Capecitabine is metabolized to 5-fluorouracil that can cause potentially fatal drug-induced toxicities. Gemcitabine is sold under the Gemzar® brand and must be administered intravenously. It is used primarily to treat pancreatic, breast and non-small cell lung cancer. It works by inhibiting DNA synthesis.
This portfolio creates a new cancer-treatment via a drug that is a nucleoside derivative – a hybrid of Capecitabine and Gemcitabine. This new formulation is designed to avoid metabolism to 5-fluorouracil and its associated toxicities such as dose-limiting hand-foot syndrome. It causes DNA damage by analog misincorporation into the DNA without being chemically reactive, unlike most DNA-targeted anticancer drugs such as alkylating agents, platinum drugs, and others. A prototype covered by this portfolio was synthesized and tested for biological activity. The results showed significant anticancer activity both in vitro and orally in vivo, more potent than Capecitabine alone. The damage caused to the DNA by this new treatment leads to the death of the cancer cells by apoptosis. By attacking multiple cancer targets with different mechanisms, this patented compound reduces the likelihood of the resistance to the cancer-fighting drug – a major drawback of nucleoside-based anticancer and antiviral drugs. Accordingly, a single compound covered by this portfolio can achieve the effects of a combination of two drugs – such as Capecitabine and Gemcitabine – with the oral administration of a single compound with a single pharmacokinetic profile.
Patent Portfolio
This portfolio would be a strategic acquisition for any pharmaceutical manufacturer in the oncology sector.
Xeloda is a registered trademark of Hoffman-La Roche, Inc.
Gemzar is a registered trademark of Eli Lilly & Company.
 Pre-Filled Syringe (Balda Medical): U.S. and European Patent Portfolio
Pre-Filled Syringe (Balda Medical): U.S. and European Patent Portfolio
The syringe has been in use in medicine for almost 200 years. Connected to a hypodermic needle, it is used to inject liquid-form medicine into humans and animals, and to withdraw fluids such a blood or venom. To administer medicine, the standard procedure is to attach a hypodermic needle to a syringe, push the needle into a vial that containers the medicine, pull back on the plunger (or “piston” as it is also known), and draw the medicine into the syringe. The needle is then inserted into the patient, and when the piston is pushed down, the medicine is injected into the patient via the needle. But wait. There are many drugs and supplements that are taken by injection on a regular basis – these include insulin, blood thinners, pain remedies, cancer medications, fertility drugs, and anti-body blocking drugs for those with allergic diseases. There are also supplements such as Vitamin B that are taken at home by injection on a regular basis. If a patient is going to take the same medication on a regular basis, wouldn’t it be convenient if the syringe were shipped pre-filled with the insulin or other medication?
Those medications and supplements that are currently available in a syringe are shipped without the piston inserted in the syringe to prevent the contents of the syringe from being accidentally ejected from the cylinder of the syringe during shipping. To prevent his from happening, the piston is shipped in a separate package, and that is costly in terms of packaging and transportation costs. And if either package gets separated from the other, the user is stranded. This portfolio covers a pre-filled syringe that includes a receiving tube with a nozzle and a piston. The piston is inserted over the end of the receiving tube on the nozzle side. The design creates a pre-filled syringe that is delivered along with the piston as one convenient unit.
This portfolio consists of U.S. Patent No. 9,592,334 for a “Pre-fill syringe” and European Patent 2383005 for a “Filling injection device.” The European Patent is valid in Germany, the UK, France, Italy, Switzerland, and Spain. Any company that sells medicine or supplements that are taken by injection on a regular basis, or any pharmaceutical packaging manufacturer, can use this portfolio to create a highly convenient pre-filled syringe product.
 Smart Drug Dispenser (Pill Development Group): U.S. Patent Portfolio
Smart Drug Dispenser (Pill Development Group): U.S. Patent Portfolio
Opioid addiction has become an epidemic in the U.S. Many patients who were prescribed opioid drugs to treat pain became addicted. Others illegally acquire opioid drugs. The result is the same – ruined lives and overdose deaths – over 100,000 in 2021! In addition to the opioid crisis, there are all the Americans – especially older citizens – who are on prescription drugs, but forget to take a prescribed drug, or forget they took the drug and accidentally double-dose. There has to be a technology by which a prescribed drug is made available to a patient when he or she needs it, and at no other time. A technology that does NOT enable a patient – accidentally or purposely – to overdose!
This portfolio addresses this issue head-on by creating a smart pill dispenser that will hold any number of pills to match a prescription – 30, for example, if the pill is to be taken once a day for one month – that are held in a two-piece clamshell cover. At a time interval determined by the prescription, a motor unlocks the container and it can be advanced to expose the next dose of medication. The patient takes that pill and nothing happens until it is time for the next dose. The patient cannot advance to the next pill until the time interval has passed, so he or she cannot overdose. While the technology applies to any prescription drug, it is especially valuable to patients taking potentially addictive drugs as it prevents them from taking more than the prescribed dose. Additionally, data capture of the usage of potentially addictive medications can be used to determine prescribing patterns. Better packaging of prescribed medications can also prevent children from having access to an entire bottle of medication with disastrous consequences.
Patent Portfolio
This portfolio will enable a drug packaging or pharmaceuticals manufacturer to offer a practical, affordable, and effective solution to overdosing of opioid and other addictive drugs, as well as packaging that will help older patients keep track of the drugs they took and need to take, and prevent a drug from being taken by children. The portfolio also includes claims that significantly reduce manufacturing costs. Proof-of-concept models are available upon request from the invention team.
 Novel Molecule for Treatment of Central Nervous System Diseases (Montisera): International Patent Portfolio
Novel Molecule for Treatment of Central Nervous System Diseases (Montisera): International Patent Portfolio
A new drug candidate – known as the “D‑15” molecule – has the potential to treat diseases of the Central Nervous System. This includes dependency disorders such as alcohol utilization disorder, drug addiction, anorexia, and bulimia, as well as disorders of high complexity including Parkinson’s Disease, depression, narcolepsy, hyperactivity, and Alzheimer’s disease.
Extensive Pre-Clinical Testing has been conducted for the D‑15 molecule including heart safety, acute toxicity, alcohol utilization invivo efficacy, primary pharmacodymamics, safety pharmacology, pharmacokinetics ADME, toxicology, specification (LCMS, NMR, IR, and UV/VIS), bioavailability, and Parkinson’s Disease invivo. Based on pre-clinical trials, the molecule has a promising toxicity profile.
Patent Portfolio
This portfolio creates the opportunity for a pharmaceutical manufacturer to bring to market a drug for the effective treatment of multiple Central Nervous System disorders. A detailed account of the pre-clinical trials, and a complete list of the patents in the portfolio, are included in the Prospectus. In addition to the patent, and the Pre-Clinical Testing data, this portfolio includes 50-100g of the synthesized drug for further trials and testing.
 Flavocillin – Next-Generation, Non-Resistant Penicillin Derivative: European and PTC Patent Applications
Flavocillin – Next-Generation, Non-Resistant Penicillin Derivative: European and PTC Patent Applications
Since penicillin was discovered almost 100 years ago, it has been the Wonder Drug of the 20th Century. However, over time the bacterial infections treated by penicillin built up a resistance to the drug. So, science has needed to keep itself a step ahead of the each new bacterial strain by developing penicillin derivates such as ampicillin, azlocillin, carbenicillin, mezlocillin, piperacillin, and ticarcillin. What is clearly needed in a penicillin-based antibiotic against which bacterial strains cannot build a resistance!
This portfolio addresses that need. Flavonoid is a substance that strengthens the immune system when taken into the body, and it is also antimicrobial. However, against infections its structure is not enough on its own. To be a truly effective antibiotic, synthesis had to be done to create Flavocillin, a penicillin derivative. In addition to being highly effective, Flavocillin has far fewer side effects compared to many other antibiotics and anti-infective agents.
Flavocillin is highly effective against enterecoccus hirae, hepatobiliary infections, endocarditis, surgical wound infection, bacteremia, neonatal sepsis, enterococcus faecalis, staphylococcus aureus, pneumonia, meningitis, osteomyelitis, endocarditis, toxic shock syndrome, bacteremia, eschericia coli, and others. There is some preliminary data showing Flavocillin to be an HIV Protease inhibitor and useful in the elimination of coronavirus symptoms.
This portfolio includes European Patent Application 3752509 for “Flavocillin: a new penicillin derivative” and PCT Patent Application 2019155266 for “Flavocillin: a new novel antibitoic that the bacteria will never develop.” The inventor is seeking an investor to fund testing of this new, exciting drug.
 Effective Treatment for Opioid Addition (Gooberman): U.S. Patent Nos. 10,568,842 and 11,033,510
Effective Treatment for Opioid Addition (Gooberman): U.S. Patent Nos. 10,568,842 and 11,033,510
Since 1999, three quarters of a million people have died from opioid overdoses in the U.S. alone. Opioid addiction is at epidemic proportions and has been exacerbated by the Coronavirus pandemic. Naltrexone and buprenorphine (sold under the Vivitrol and Sublocade brands) have been used to block the effects of opioids to treat addiction, but with limited effectiveness.
U.S. Patent No. 10,568,842 adds a steroid to naltrexone or buprenorphine preparations to create a longer lasting Medication Assisted Treatment (MAT) for opiate addiction. A continuation-in-part to this patent includes the addition of steroids to other long-acting preparations as well as steroid microcapsule preparations. Since all the ingredients in both formulations are each on their own FDA approved, approval for emergency use authorizations of the new formulation should be relatively routine and swift.
U.S. Patent No. 10,568,842 and and 11,033,510 for an “Anti-inflammatory pharmaceutical composition and methods of administration” would enable any pharmaceutical supplier to offer the next-generation of opioid addiction treatment.
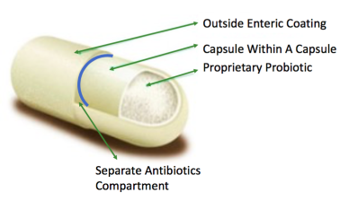 New Treatment for Bowel Diseases (Trachtman): International Patent Portfolio
New Treatment for Bowel Diseases (Trachtman): International Patent Portfolio
Three million Americans suffered from Inflammatory Bowel Disease (IBD) – either Crohn’s disease or ulcerative colitis – as of 2015, the last year for which the Centers for Disease Control has data. This is an increase from the two million cases reported in 1999. IBD is currently treated with anti-inflammatory drugs such as corticosteroids, aminosalicylates, mesalamine, and olsalazine. These are immune system suppressors that prevent the body from releasing inflammation-inducing chemicals. There has also been a rush towards biologics, which are extremely expensive with many side effects, and are not curative.
This portfolio takes a totally new approach to the treatment of Inflammatory Bower Disease. It uses a combination of antibiotic and probiotic drugs such as sulfamethoxazole/trimethoprim (SMX/TMP) and vancomycin that are far more effective than the anti-inflammatory drugs currently in use. U.S. Patent No. 9,138,441 covers a method for the treatment or prophylaxis of inflammatory bowel disease for a period of at least 120 days using sulfamethoxazole/trimethoprim with the antibiotic component releasing at a pH of 7 or greater. U.S. Patent No. 9,649,348 covers a platform of oral-dosage drug products using a pH-dependent dual-release mechanism. This patent covers not just a treatment for IBD, but also for infectious diseases, traveler's diarrhea, and Irritable Bowel Syndrome (IBS), a condition that affects more than 30 million people in the U.S.!
The use of antibiotic and probiotic drugs to treat Inflammatory Bowel Disease is well documented to have many benefits:
Patent Portfolio
This portfolio will enable a pharmaceutical company to bring to market a totally new approach to the treatment of Inflammatory Bowel Disease and Irritable Bowel Syndrome, conditions that affect 33 million Americans and tens of millions more around the world. Additional applications include pediatrics, urology, and veterinary medicine.
 Canola-Based Skin Whitener (KGK): International Patent Portfolio
Canola-Based Skin Whitener (KGK): International Patent Portfolio
Women of all ethnicities around the world have used skin whiteners for decades to reduce over-pigmentation and address skin conditions such as aging spots, liver spots, freckles, acne scars and melasma, and to even out the color of their skin, giving them a younger look. One of the most common skin whiteners is hydroquinone, but hydroquinone comes with several side effects - skin irritation and sensitization, burning, stinging, dermatitis, dryness, redness and inflammation. What is needed in the marketplace is an effective and affordable skin whitener made from natural ingredients so it has far fewer side effects.
That product now exists and it is covered by this portfolio. Using 100% natural canola extracts, this portfolio creates a next-generation skin whitener that is effective, but with NO side effects!
Patent Portfolio
The acquirer of this portfolio will be able to introduce a skin whitening product line that is affordable and effective, but with NO side effects so it can be used on a daily basis and incorporated into cosmetic products such as foundation.
 Blood Glucose Reduction (KGK): International Portfolio
Blood Glucose Reduction (KGK): International Portfolio
Metabolic Syndrome is a condition that affects over 20% of adult Americans, and three million new cases are diagnosed each year. Metabolic Syndrome includes high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels. Metabolic Syndrome significantly increases a person's risk for heart attack and stroke. If we could treat Americans with Metabolic Syndrome when it is first diagnosed, we would prevent millions – quite literally millions – of heart attacks and strokes each year!
This portfolio takes on that challenge head-on! It creates a medication consisting of limonoids (extracts from sweet or sour-scented citrus fruits), flavonoids (fruit and vegetable extracts that contain 15 carbon atoms and are soluble in water) and tocotrienols (vegetable extracts with unsaturated isoprenoid side chains with three carbon-carbon double bonds) that provides anti-diabetic and antihyperlipidemic benefits to diabetic subjects who are currently on medication but not meeting their physician’s target levels for blood glucose, Hemoglobin A1C, blood pressure and total cholesterol.
Patent Portfolio
One of the primary benefits of the composition created by this portfolio is that none of the ingredients are synthesized but are widely used natural substances. This portfolio would be a strategic acquisition for any pharmaceutical or nutritional supplement company seeking to provide a new and effective treatment for reducing blood sugar levels.
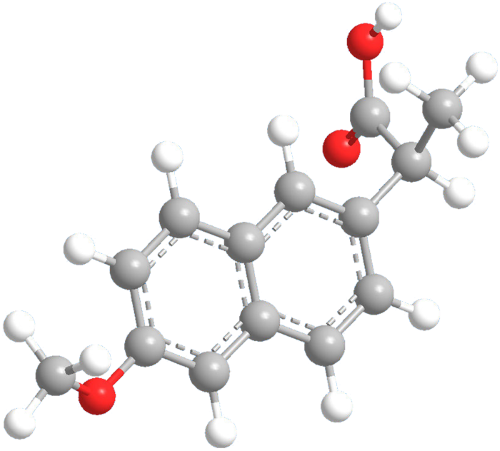 Next-Generation NSAID (Kazan): International Patent Portfolio
Next-Generation NSAID (Kazan): International Patent Portfolio
Global annual sales of nonsteroidal anti-inflammatory drugs (NSAIDs) are estimated to be over $12 billion. In the U.S. alone, over 10 million prescriptions are written each year for Naproxen and over 25 million for Ibuprofen. Naproxen is sold under various over-the-counter formulations – Aleve® being the most popular. Naproxen is an NSAID that treats pain from inflammatory diseases such as rheumatoid arthritis and joint disease as well as menstrual cramps, fever and other maladies. While Naproxen and Ibuprofen are popular and widely used, they come with numerous known side effects that include dizziness, headaches, bruising, allergic reactions, heartburn and – most often – stomach pain. The use of Naproxen and Ibuprofen is also known to increase the risk of heart disease, gastrointestinal bleeding and stomach ulcers.
This international portfolio is directed to a next-generation NSAID and has been shown in pre-clinical studies to be more effective than Naproxen and Ibuprofen as an anti-inflammatory agent, analgesic and fever reducer while having significantly less side effects than either Naproxen or Ibuprofen and being approximately 40 times less gastrotoxic than Naproxen and approximately seven times less gastrotoxic than Ibuprofen. Since this new formulation uses currently approved ingredients (Vitamin B6 and Naproxen), testing and approval for introduction of a new drug based on this formulation should be relatively routine and expeditious.
Patent Portfolio
This portfolio would be a strategic acquisition for any pharmaceutical company seeking long-term dominance in the global NSAID market.
Aleve is a registered trademark of Bayer AG.
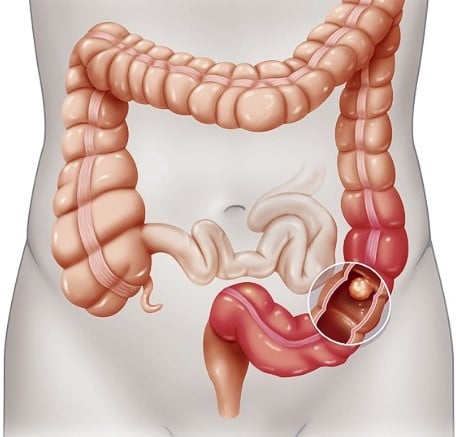 Colon Cancer Screening (Lee): Portfolio of Four U.S. Patents
Colon Cancer Screening (Lee): Portfolio of Four U.S. Patents
Despite the popularity and increased use of colonoscopies, 50,000 people will die in the U.S. from colon cancer this year. This portfolio covers a technology that replaces the traditional colonoscopy with a test that effectively detects the earliest stage of colon cancer, and also detects large and still-benign polyps. Unlike a colonoscopy, this test does not require bowel preparation or anesthesia. The technology covered by this portfolio – which is supported by several published studies – detects highly altered mRNA activity from a simple rectal smear.
| Patent No. | Title |
| 8,883,440 | Method to predict or diagnose a gastrointestinal disorder or disease |
| 9,353,420 | Method to predict or diagnose a gastrointestinal disorder or disease |
| 10,011,879 | Method to predict or diagnose a gastrointestinal disorder or disease |
| 10,400,284 | Method to predict or diagnose a gastrointestinal disorder or disease |
The company that acquires this portfolio will be able to offer an affordable test that accurately and reliably detects the earliest instance of colon cancer without the cost, time and inconvenience of a colonoscopy. The Prospectus includes the published studies that support this patented technology.
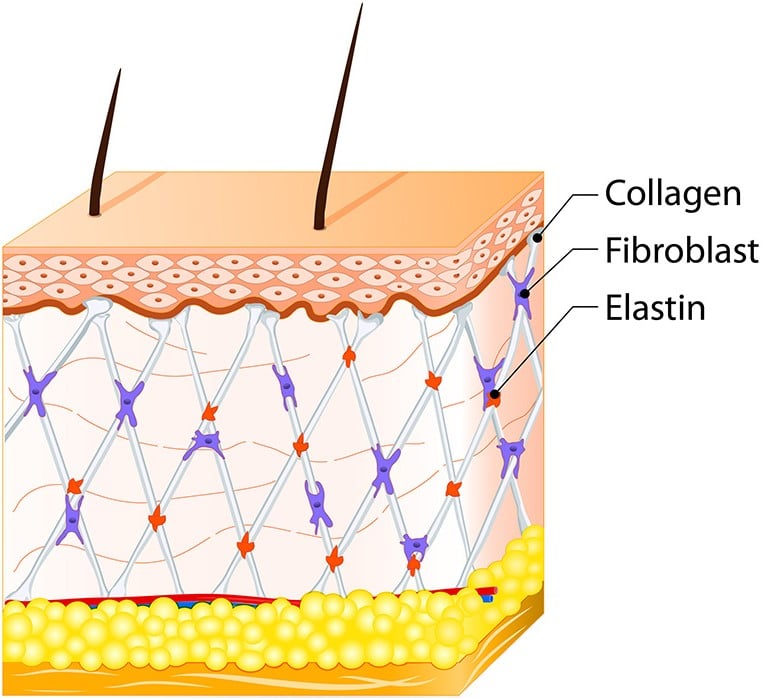 New Technology for Producing Collagen (Malinin): U.S. Patent No. 9,737,590
New Technology for Producing Collagen (Malinin): U.S. Patent No. 9,737,590
Collagen is used in cosmetic surgery, wound care, filling tissue voids and for regeneration of damaged structure. This patent describes a method of producing pure collagen from source material of various tissues – both human and animal – that can be used for a variety of therapeutic and cosmetic applications. The technology covered by this patent enables collagen fibers to be separated from freeze-dried tissues by a magnetic field in a liquid medium. This method induces collagen fibers to self-assemble and produce gels, paste membranes and various other configurations. An additional benefit is that the patented process does not depend on harsh chemical treatment such as hydrolysis or harsh physical treatment. This technology is superior to currently available techniques because it can utilize human tissues as source material and because it can produce collagen without cross-linking.
U.S. Patent No. 9,737,590 for "Self-assembly of collagen fibers from dermis, fascia and tendon for tissue augmentation and coverage of wounds and burns" would be a strategic acquisition for any biomedical or pharmaceutical company that is currently producing regeneration products as it would give this company a critical competitive advantage by enabling it to offer a new and unique variety of collagen products.
 Alternate Treatment for Colon Cancer (System C): International Patent Portfolio
Alternate Treatment for Colon Cancer (System C): International Patent Portfolio
Until just recently, all cancer treatments were either chemotherapy or radiation. This portfolio covers a non-chemotherapy treatment for colon cancer that uses the cognitive ability of the brain to fundamentally cure a disease. The inventor – a physician – tested the concept on his patients with good results. The patents in the portfolio call for pre-surgery administration over a period of days of haloperidol, ascorbic acid, calcium pantothenate, and a solution of maltose, potassium chloride, magnesium chloride, potassium hydrogen phosphate, potassium dihydrogen phosphate, sodium chloride, magnesium chloride hexahydrate, anhydrous sodium acetate and maltose monohydrate. That is followed with Luvox and sodium ferrous citrate if the patient is anemic. This treatment, pre-surgery, enhances the cognitive ability of the brain to guide the body’s organs and systems to cure itself from cancer.
Patent Portfolio
The PCT Application is currently inactive, but it could re re-activated and used to secure additional, national patents. Most importantly, the specific medications that comprise the total treatment have either no or very minor side effects, so the prescribed treatment is a Low Risk/High Reward proposition! This portfolio would be a critical acquisition for any pharmaceutical company!
Treatment of Cerebral Palsy and Multiple Sclerosis (Gilrose Pharmaceuticals) Twelve U.S. Patents, Two PCT Patent Applications and One U.S. Patent Application
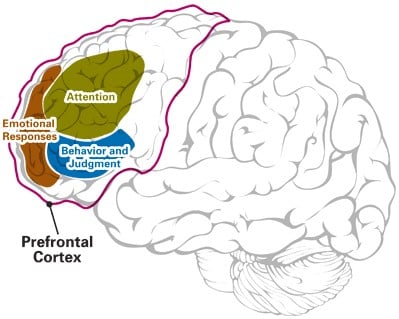
This portfolio covers a new application for an existing drug for the treatment of brain disorders known as Pre-Frontal Processing Disorder (PFD). This condition is defined by the inability of the pre-frontal cortex to integrate sensory input with motor output. This drug can be used to treat Cerebral Palsy, Multiple Sclerosis, and Autism-spectrum sufferers with Speech Apraxia and Gait Disorder.
| Patent No. | Title |
|---|---|
| 8,883,815 | Treatment for cerebral palsy impaired speech in children |
| 9,089,563 | Method of treating apraxia of speech on children |
| 9,155,502 | Treatment for cerebral palsy impaired speech in children |
| 9,161,718 | Treatment for cerebral palsy impaired speech in children |
| 9,220,712 | Pharmaceutical intervention and method for treating an apraxia of speech in children |
| 9,307,942 | Treatment for cerebral palsy gait impairment |
| 9,333,198 | Treatment for cerebral palsy gait impairment |
| 9,375,421 | Pharmaceutical intervention for treating an apraxia of speech in children |
| 9,408,838 | Treatment for cerebral palsy gait impairment |
| 9,682,073 | Pre-frontal cortex processing disorder gait and limb impairments treatment |
| 10,085,414 | Pre-frontal cortex processing disorder gait and limb impairments treatment |
| 10,420,318 | Pre-frontal cortex processing disorder gait and limb impairments treatment |
| Application No. | Title |
| WO2015061125 | Treatment of cerebral palsy impaired speech in children |
| WO2017007577 | Pre-frontal cortex processing disorder, gait and limb impairment treatment |
| 20190320611 | Pre-frontal cortex processing disorder gait and limb impairments treatment |
The acquirer of this portfolio will have to invest in clinical trials, but it will have a drug it can take to market to treat over 100 million sufferers worldwide, and it can use the PCT Patent Applications to secure patent coverage in over 100 countries for global patent coverage.
Patent Brokerage Prospectus: Contact [email protected] to receive an analysis of each portfolio that includes:
We offer patents in these technologies:




